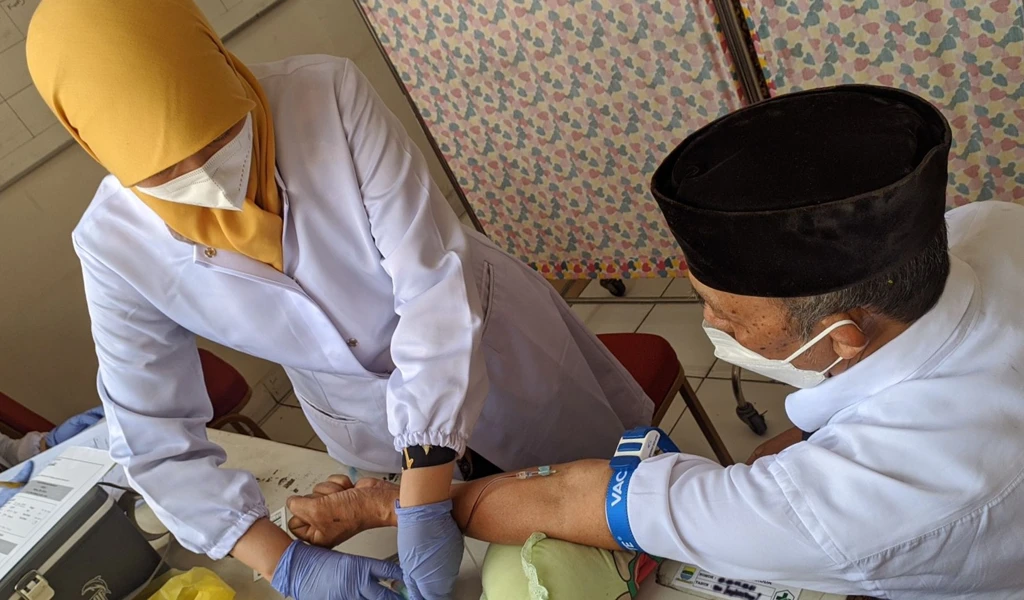
CEPI-funded COVID-19 vaccine candidates progress to clinical trials
CEPI's COVID-19 vaccine programme with Clover Biopharmaceuticals began phase 1 clinical trials on Friday (19 June) in Perth, Australia, to test the safety and immunogenicity of their protein-based vaccine candidate.
Clover becomes the fifth CEPI-funded vaccine candidate to begin clinical testing—alongside our partners Moderna Inc., Novavax Inc., University of Oxford and AstraZeneca, and Inovio. Of the 224 vaccine candidates in development around the world, 5 out of the 15 COVID-19 vaccine programmes now in clinical trials have received funding from CEPI.
Last week also saw CEPI partner CureVac receive regulatory approval for its COVID-19 vaccine candidate and the University of Queensland started recruiting volunteers for its upcoming Phase 1 trial.
Vaccines represent our best exit strategy from the COVID-19 pandemic. No other single measure will have a similar impact on the world's ability to respond to this threat.
When building our portfolio, CEPI has focussed on COVID-19 vaccine candidates that have the potential to be developed at speed, at scale and made globally accessible.
Last week's developments are indicative of the incredible and rapid work being undertaken by our partners, which is unprecedented in the field of vaccine development. We still have a long way to go, but with this level of collaboration and innovation, we are continuing to move as quickly as possible in the hope that we can reduce the time needed to bring an end to this devastating crisis.
In total, 150 human volunteers are expected to be enrolled in Clover's study to test the safety and immunogenicity of its COVID-19 S-Trimer subunit vaccine candidate. The Phase 1 trial will also evaluate two adjuvants, produced by Dynavax and CEPI partner GSK, which aim to enhance the immune response and reduce the amount of antigen required per vaccine dose.
CEPI first partnered with Clover Biopharmaceuticals in late April, providing up to US $3.5 million, to support preparation and initiation of a trial with their protein-based candidate vaccine.
Preliminary safety and immunogenicity results are expected in August 2020, working towards CEPI's ambition to have at least three vaccine candidates that could be submitted to regulatory authorities for licensure for general use within an accelerated 12-18 month timeframe. If successful in clinical trials, Clover Biopharmaceuticals has the capacity to rapidly scale-up the production of the vaccine using its in-house biomanufacturing facilities.
On June 17, CureVac also announced that it had received regulatory approval from German and Belgian authorities to initiate Phase 1 clinical trials of its mRNA COVID-19 vaccine candidate. CEPI first invested in CureVac mRNA vaccine platform in 2019. In January, 2020, CEPI provided up to US $15.3 million to extend the partnership to advance their mRNA COVID-19 candidate. First trial participants will be vaccinated at the Institute for Tropical Medicine in Tübingen and the Ghent University Hospital (Belgium), the Tropical Institute of the University Hospital Munich, LMU (Germany), and the Hannover Medical School (Germany).
Meanwhile, the University of Queensland (UQ) have begun recruiting for healthy volunteers for its upcoming first in-human study. Following initial funding in January, CEPI recently extended this partnership to include CSL to carry out industrial-scale manufacturing of UQ's "molecular clamp" enabled COVID-19 vaccine candidate to allow for the production of potentially millions of doses a year, should the product be approved. Additional funding contributions will also be used to provide support for the pending phase 1 study and subsequent late-stage clinical trials.
CEPI remains committed to global equitable access across its COVID-19 vaccine programmes. It is anticipated that vaccines produced under the CEPI portfolio will be procured and allocated through the COVID-19 Vaccine Global Access Facility, an instrument of the Vaccines pillar of the Access to COVID-19 Tools (ACT) Accelerator, which aims to ensure equitable access to COVID-19 vaccines for all countries, at all levels of development, that wish to participate.
The ACT Accelerator is an international initiative, set up by the World Health Organization and global leaders in April, to accelerate the development, production and deployment of safe and effective diagnostics, therapeutics and vaccines against COVID-19 — making them accessible to everyone, worldwide.
______________________________________________________
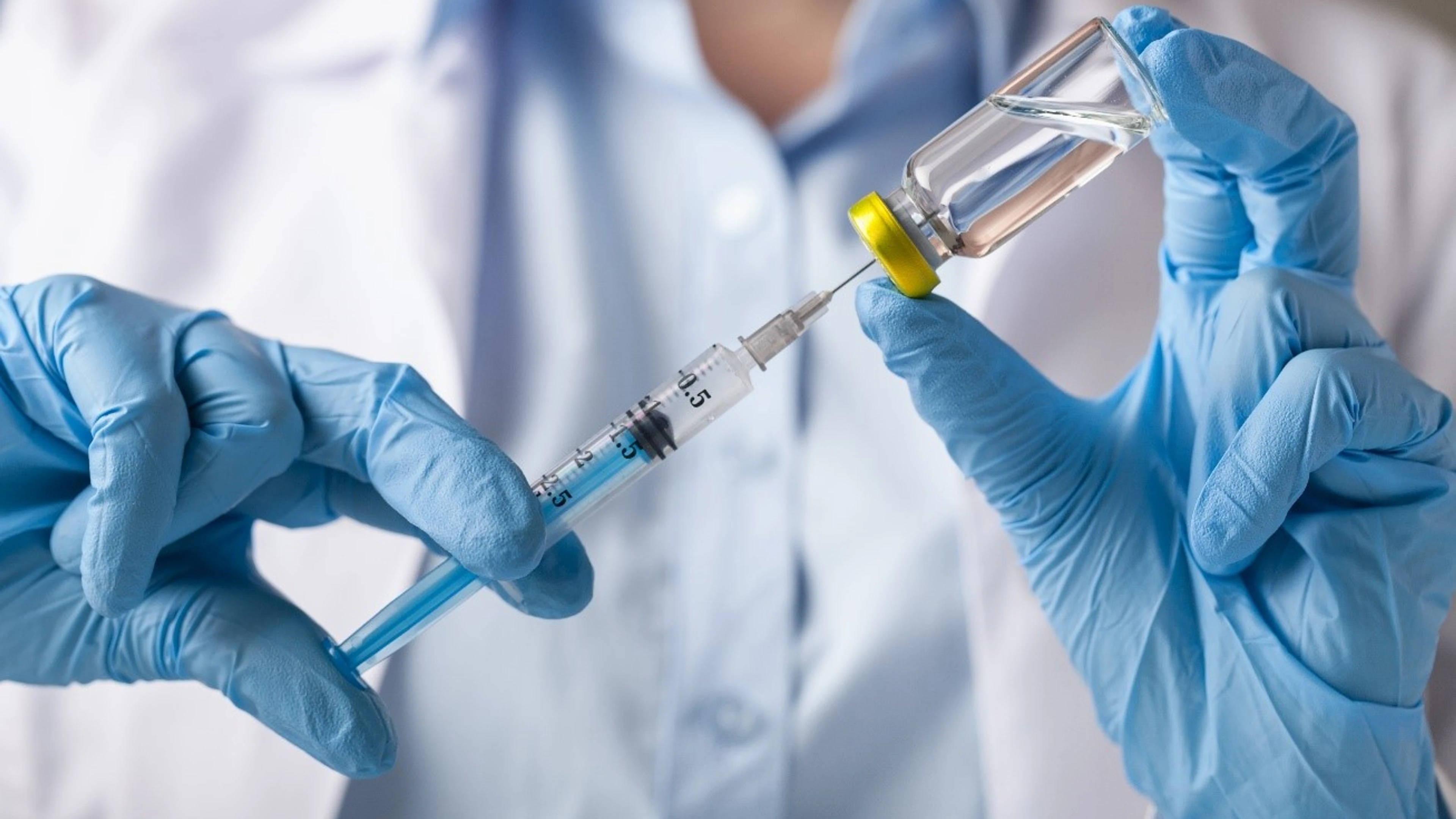
Photo caption: Hand holding syringe and vaccine. Credit: Billion Photos/ Shutterstock.