The COVID-19 vaccine R&D landscape has developed at unprecedented scale and speed since our initial analysis in April identified 115 candidates in the pipeline (Nat. Rev. Drug Discov. 19, 305–306; 2020). In this updated overview, we focus on candidates in clinical trials and provide some initial perspectives on their clinical development.
Evolution of the R&D landscape
As of 3 September 2020, the global COVID-19 vaccine R&D landscape includes 321 vaccine candidates, an increase of more than 2.5 fold compared with our previous report. Of these, 33 vaccine candidates are in clinical trials (Supplementary Table 1), with plans to enrol more than 280,000 participants from at least 470 sites in 34 different countries. The most advanced clinical candidates are now in phase III trials, and data to support licensure are anticipated to be available later this year. For the leading candidates, large-scale manufacturing of vaccine has already been initiated to enable rapid distribution if approval is obtained.
Technology platforms and targets. The current COVID-19 vaccine pipeline comprises a broad range of technology platforms, including both traditional and novel approaches (Fig. 1). Early data are emerging for the most advanced clinical candidates, and although encouraging antibody and T cell responses have been reported for vaccines based on several of the different platforms being used, it is too early to assess their relative potential. Twelve clinical-stage vaccine candidates employ adjuvants.
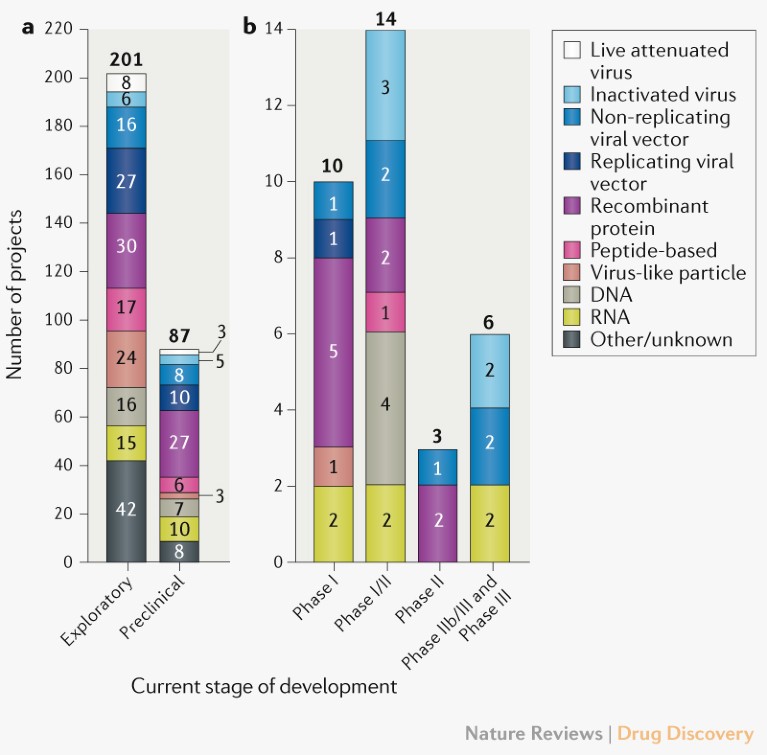
Fig. 1 | Pipeline of COVID-19 vaccine candidates by phase of development and technology platform. a | Exploratory and preclinical pipeline. b | Clinical pipeline. Traditional approaches include live attenuated and inactivated; novel approaches include viral vector, RNA, DNA, recombinant protein, peptide-based, virus-like particle. See Supplementary Box 1 for details of the data set and analysis.
The majority of vaccine candidates currently in clinical trials target the spike (S) protein and its variants as the primary antigen. However, candidates that target other or multiple antigens are progressing, including candidates that target N protein, attenuated vaccines, inactivated vaccines and peptide vaccines (Supplementary Figure 1).
Vaccine developers. The biggest change in the overall profile of COVID-19 vaccine developers (Fig. 2) since April has been the increasing engagement of large multinational companies. Of the candidates currently in the clinic, eleven are being developed by Chinese organizations, and seven are being supported by the US Operation Warp Speed programme, which aims to deliver 300 million vaccine doses for COVID-19 by January 2021 and has so far announced funding of more than US$10 billion to advance vaccine development. Eight of the clinical candidates have received funding from the Coalition for Epidemic Preparedness Innovations (CEPI) and are now included in the portfolio of COVAX, a collaboration led by CEPI, Gavi and the WHO that aims to deliver two billion vaccine doses for global allocation by the end of 2021.
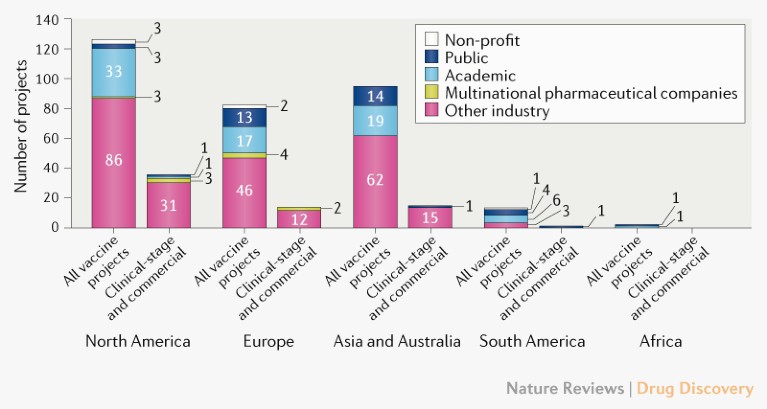
Fig. 2 | Profile of COVID-19 vaccine developers by type and geographical location. The ‘other industry’ category includes companies other than those in the ‘multinational pharmaceutical company’ category, which are defined as having revenues of more than US$10 billion per year. For partnerships, the location is that of the lead developer. See Supplementary Box 1 for details of the data set and analysis.
Perspectives on clinical development
The progression of COVID-19 vaccine candidates into clinical development is beginning to lead to insights that may be useful for informing future COVID-19 vaccine development efforts, as well as vaccine R&D strategies for future outbreaks. The WHO has also released a target product profile for COVID-19 vaccines, which provides guidance for clinical trial design, implementation, evaluation and follow-up. Some of the most important considerations for clinical development of COVID-19 vaccine candidates are briefly summarized below.
Trial design. An accurate estimate of the background incidence rate of clinical COVID-19 end points in the placebo arm is required for a robust sample size calculation in a conventional clinical trial. However, the rapidly changing epidemiology of the COVID-19 pandemic means that it is challenging to predict incidence rates, and trial design is further complicated by the effect of public health interventions to help control the spread of the virus, such as social distancing and quarantine. Thus, an adaptive case-driven trial design, in which power and precision are not determined by the size of the trial but rather by the overall number of COVID-19 cases identified for the primary end point, is worth considering. Recruitment is discontinued when the minimum necessary number of events is reached, resulting in a more efficient, effective and rapid clinical trial.
Clinical end point. It is crucial to choose an end point that is likely to reflect the desired relevant public health benefit. Possible end points for consideration in COVID-19 vaccine trials include clinical disease of varying severity and/or asymptomatic infection. Vaccines for respiratory and other mucosal viruses historically have greater efficacy against more severe rather than milder disease and are less likely to affect asymptomatic infection. In addition, use of asymptomatic SARS-CoV-2 infection as an end point may be operationally challenging and result in a large number of false-positive test results and possibly even failure to demonstrate vaccine efficacy. By contrast, use of a clinical end point requiring signs or symptoms of pneumonia may result in earlier demonstration of vaccine efficacy, as this limits the number of cases of vaccine-induced disease attenuation (that is, people with SARS-CoV-2 infection but only mild, residual clinical symptoms following vaccination) included in the primary efficacy analysis. Thus, the rate of occurrence of the end point in the population under consideration, the importance of the vaccine’s impact on the end point and the reliability in measuring the end point should be considered when defining an end point.
It is important to maintain some flexibility in the clinical end point definition in a pandemic situation involving a novel pathogen because there is limited knowledge of pathogen-specific disease presentation and underlying pathophysiology. This flexibility enables the collection of clinical case data in early-stage clinical trials, with vaccine efficacy being established in later-stage trials using an evolved case definition based on emerging knowledge. Infection or disease end points not included to address an efficacy trial primary objective should, however, be assessed as secondary end points.
Correlates of protection. What constitutes protective immunity for those exposed to SARS-CoV-2 remains unclear. However, emerging data are indicating that both neutralizing antibodies and cell-mediated immune responses are important in the response to SARS-CoV-2, and potential vaccines should induce both of these responses.
Target population. Relaxing the eligibility criteria to broaden the trial population is of key importance for advanced-stage COVID-19 vaccine trials. The population studied should be representative of the wider population in which the vaccine will be used, and every effort should be made to recruit strategically to demonstrate vaccine efficacy as early as possible. Thus, adequate representation of populations at risk of SARS-CoV-2 infection and/or severe consequences — such as frontline health-care workers, elderly people and those with underlying health conditions — is encouraged, as they may benefit most from a safe and effective vaccine. Vaccine construct-specific characteristics should be taken into account.
Safety considerations. Development of an adequate safety database is crucial for regulatory approval and public acceptance of any new vaccine, especially one using a novel technology platform. Harmonization of safety data collection across vaccine candidates maximizes their comparability and value. Towards this end, the following tools, many recommended by the WHO, are or will be available soon: >60 standardized case definitions for adverse events following immunizations (AEFI); a list of potential adverse events of special interest (AESI) for COVID-19 vaccines, their case definitions, implementation tools and some background rates; standardized templates for collection of key information for benefit–risk assessment of vaccines by technology, including nucleic acid, protein, viral vector, inactivated viral and live viral vaccines; and outcomes from a consensus meeting on vaccine-mediated enhanced disease (Vaccine 38, 4783–4791; 2020).
The traditional standards for sample size, duration of follow-up and heterogeneity of study populations in pre-licensure trials may have to be adapted, in close coordination with regulators to ensure safety and efficacy, to meet the unprecedented need for rapid development of COVID-19 vaccines. Furthermore, near-simultaneous global introduction of multiple vaccines, many with novel technologies, is planned. Hesitancy to receipt of COVID-19 vaccines is also emerging, and so planning for and implementation of a robust global community engagement and post-introduction pharmacovigilance system is urgently needed.
Outlook
Although the leading COVID-19 vaccine candidates have progressed to advanced stages of clinical development at exceptional speed, many uncertainties remain given the lack of robust clinical data so far. Moreover, given the highly unusual circumstances associated with developing a vaccine during the evolution of a novel global pandemic, probability of success benchmarks for traditional vaccine development are likely to underrepresent the risks associated with delivering a licensed vaccine for COVID-19. The most advanced candidates are expected to begin reporting data from pivotal studies over the coming months, which if positive will be used to support accelerated licensure of the first COVID-19 vaccines. Such data will also provide valuable insights for the field and inform ongoing and future development activities aimed not only at controlling the current global pandemic, but also for effective long-term immunization strategies against the disease.
