The vaccines and the variants: CEPI's work to tackle an evolving threat
The emergence of SARS-CoV-2 variants — including notable examples first identified in the UK, South Africa, Brazil, and now India - underscores the urgent need to double down our efforts to bring COVID-19 under control through the deployment of safe and effective vaccines globally.
Just sixteen months on from publishing the first genomic sequencing data of the novel coronavirus, SARS-CoV-2—the virus behind the COVID-19 disease—we have seen extraordinary progress in the field of vaccinology and medicine more generally.
More than one billion COVID-19 vaccine doses have now been rolled out worldwide under temporary use authorisation or emergency use licensure. This includes nearly 60 million doses through COVAX, our global initiative, co-led by CEPI, Gavi, the Vaccine Alliance and the World Health Organization (WHO), with key delivery partner UNICEF, to enable equitable access to COVID-19 vaccines to end the acute phase of the pandemic.
These major advancements in vaccine R&D and the fast-paced rollout come at a critical time in the progression of the global crisis. The virus is continuing to rapidly spread in many regions around the world, as we have seen in recent weeks in India and Brazil with their health systems operating at maximal capacity. In fact, more cases of COVID-19 have recently been reported in a two-week period than in the first six months of the pandemic. India has continued to break records, surpassing 400,000 confirmed cases a day, for several days, last week.
Could SARS-CoV-2 variants be behind the increase in cases?
There is currently concern that novel variant B.1.617, first identified in India, and the P.1 variant, first identified in Brazil, are partly behind the spike in cases in the associated countries.
While all viruses mutate over time and the vast majority of changes to the virus's genetic code will have little impact, preliminary studies suggest B.1.617 appears to be more transmissible (making it ‘easier' to spread within a population). This is because of a mutation it carries called L452R, which allows the virus's spike protein to ‘unlock' and gain entry into our cells, helping it to evade the immune system. Likewise, according to data collected in Manaus, Brazil, the P.1 variant could be twice as transmissible as earlier strains.
Research also suggests that other variants, including B.1.1.7, first reported in the UK, and B.1.351, originally identified in South Africa, may show signs of increased transmissibility, hence potentially contributing to the surge in cases worldwide.
CEPI has been working with its partners to monitor the global rise and movement of these variants. To date, nearly 1.5 million SARS-CoV-2 viral genome sequences have been made publicly available, enabling real-time progress in the understanding of the origins, geographical spread, circulation, and evolution of the SARS-CoV-2 virus.
Scientists are using this virus tracking data to better inform our understanding of the variants and guide our global response. In addition to assessing whether these variants may impact transmissibility, research is also measuring whether variants could: impact the severity of symptoms and illness in those infected, reduce immunity and allow for re-infection, and/or impact the efficacy (the degree to which a vaccine prevents disease, and possible also transmission) of COVID-19 vaccines and other countermeasures.
Early data against these properties is then being used by health agencies to designate novel variants to one of several categories. This includes variants of interest (strains known to have mutations that may help them evade antibodies or bind more tightly to human cells and cause sustained community transmission in a local are or in multiple countries) and variants of concern (strains that are demonstrated to be more transmissible, cause worsened disease and/or be resistant to available diagnostics, vaccines or therapeutics). Variants B.1.1.7, P.1, B.1.351, and, most recently, B.1.617 have been classified as variants of concern.
What about their impact on vaccines?
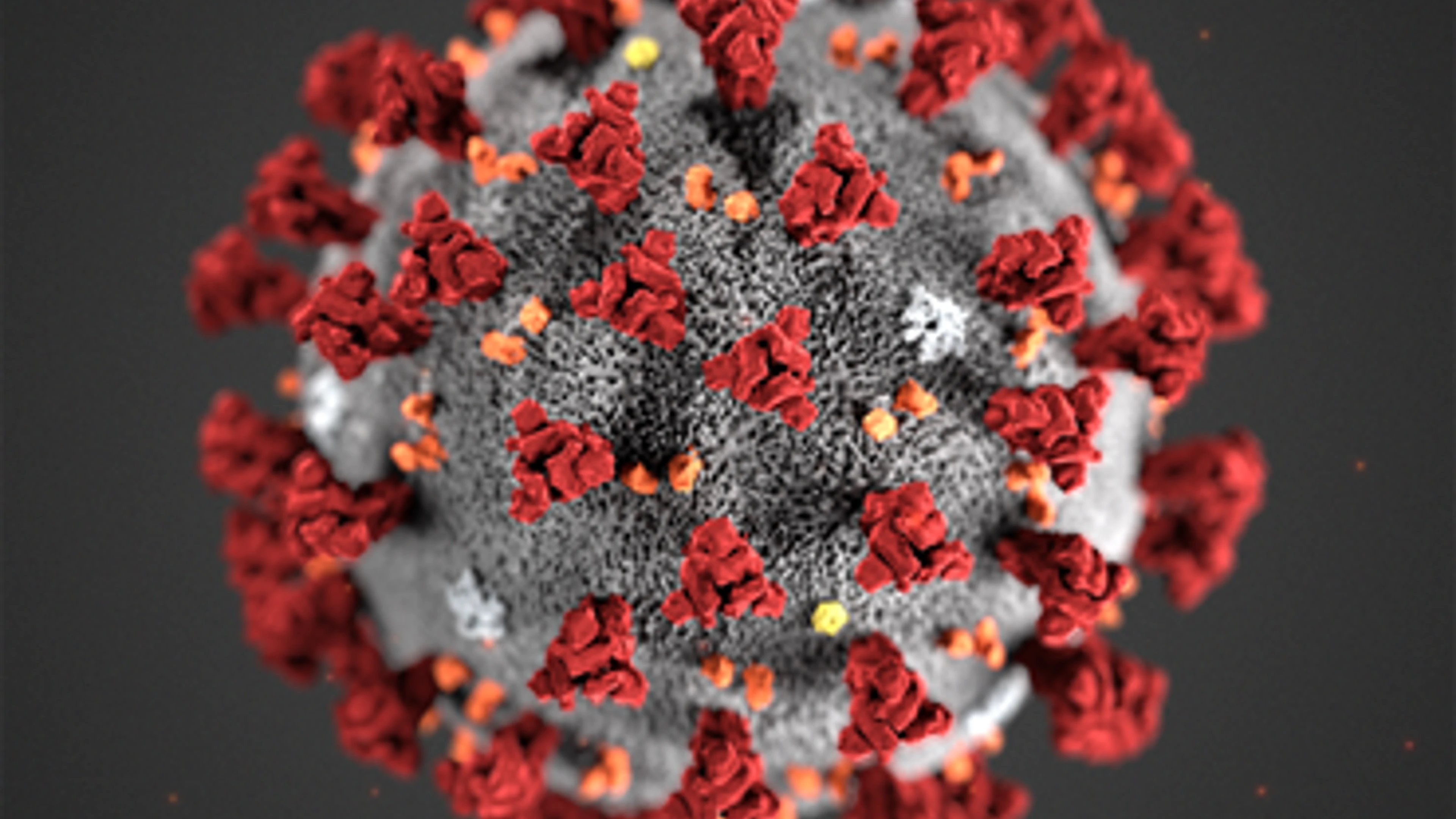
Credit: US CDC, Alissa Eckert
Researchers are particularly interested in the extent of changes to the spike protein of SARS-CoV-2 variants as this is a key target for many of the vaccines currently being rolled out and/or undergoing clinical trials.
Commonly depicted in some of the leading imagery of the virus over the past year, the spike protein is made up of 1273 amino acids (building blocks) which ‘protrude' from the main SARS-CoV-2 virus body. It is the part of the virus that antibodies—produced as part of the immune response—respond to after infection or vaccination. If changes to the spike protein are significant, antibodies may be less effective in recognising and therefore responding to (known as "neutralising") the virus, potentially rendering our vaccines less effective.
In November 2020, CEPI launched a taskforce with the GISAID Initiative and two UK laboratories—Public Health England (PHE) and the National Institute for Biological Standards and Control (NIBSC)—to not only track but also test the impact of viral variants on COVID-19 vaccine development.
This work is being completed in real-time, therefore meaning that we can quickly look to see if serum known to neutralise the original SARS-CoV-2 virus remains effective against new variants. If there are any significant changes shown through our monitoring and testing work in the pathogenesis (disease progression) and virulence (infection severity) associated with the new variant, these findings will be communicated through CEPI, WHO, and COVAX networks to developers to guide their vaccine programmes.
Preliminary research, carried out by laboratories worldwide, has found evidence that some novel variants could partially evade vaccine immunity. For example, real-world data in Israel—a country with high COVID-19 vaccination coverage—found that some individuals became infected with the B.1.351 variant despite having received one or two doses of vaccine. A second mutation of the B.1.617 variant, called E484Q, could also be of concern, as similar mutations are thought to change the spike protein in a way that makes pre-existing antibodies less likely to recognise and therefore neutralise the virus.
While it currently looks like vaccines developed will remain fully or partially effective, there may come a need to modify vaccine formulations to incorporate protection against these new strains. This would not only cost money and time, but could also prolong the global crisis further, reversing the progress of both national vaccine rollouts and COVAX.
Making ‘variant-proof' vaccines of the future
With the potential for variants to evade our vital pandemic-ending tools, CEPI, in addition to its monitoring and vaccine analysis work, is staying one step ahead through directly funding further research and development of COVID-19 vaccines tailored to target variants of concern.
In March 2021, we announced that we would provide up to $33m in funding to VBI Vaccines to develop their enveloped virus like particle (eVLP) vaccine candidate through Phase I clinical development against SARS-CoV-2 variants, including the B.1.351 variant. In addition, CEPI is also investing up to $14.2m in SK Bioscience's recombinant protein vaccine candidate to support its adaptation against variants of concern. Further funding is advancing SK Bioscience's manufacturing methods to enable the potential production of hundreds of millions of doses of its candidate vaccine.
If both vaccines are successful, VBI and SK bioscience have agreed that the vaccines supported by CEPI will be made available to COVAX for allocation to vulnerable populations worldwide. The shots could be used to increase initial supplies of vaccines and/or as potential booster doses.
Considering the threat of additional variants and potential future outbreaks caused by other coronaviruses, CEPI has also announced that it will provide up to $200m to vaccine developers to advance work to move away from the traditional "one bug, one drug" approach and create an all-encompassing coronavirus shot. Open worldwide to organisations with expertise in vaccine development, CEPI plans to invest in promising multi-coronavirus vaccine candidates up to clinical proof-of-concept.
While an incredibly challenging area of work, a broadly protective coronavirus vaccine could provide significant benefits, through potentially helping to avert another COVID-19-like pandemic, minimising the need to continually update vaccine components, and ultimately reducing virus circulation.
The work forms part of CEPI's long-term $3.5bn investment strategy, announced in March, 2021, to tackle the imminent threat of COVID-19, while also advancing the innovative tools and networks to reduce or even mitigate future pandemics and epidemics.
Additional R&D needed to win the race
Now, more than ever, we are in a race against the virus.
While there is still much to learn about the novel SARS-CoV-2 variants, it remains critical that the public comply with recommended hygiene and physical distancing measures and get vaccinated as soon as a shot is made available to them. This is to both reduce the immediate risk to human health and to slow down the rate of transmission, and by extension decrease the rate of further mutations of the virus.
The evolution of the virus also makes clear the critical importance of continued investment in tracking new mutations and further R&D on ‘variant proof' COVID-19 vaccines and more broadly protective tools. This work, led by CEPI and others, is essential if we are to equip the world with the scientific innovations that will successfully bring an end to this and future crises.
Disclaimer: This article was first published on the CEPI website on 11 May 2021. Information provided within the article may be subject to change as the COVID-19 pandemic continues and/or as more research is undertaken and made available / published which sheds light on our understanding of SARS-CoV-2 variants.